Study of the Carbothermal Reduction of Self-Reducing Briquettes Developed with Iron Ore Fines, Charcoal and Silica Fume Residues (2)
3. Results and Discussion
3.1. Sample Characterization of Wastes
Table 4 shows the size distribution, percentage of passing material (10, 50 and 90%) and the diameter (μm) of the amorphous silica fume particles, in accordance with the results reported in several works.
Table 4. Granulometric analysis performed on the silica fume.
| % Passing | Diameter (μm) |
| 10 | 4.19 |
| 50 | 17.9 |
| 90 | 48.1 |
| Medium diameter | 23.3 |
Figure 4 shows the particle size distribution curve with the accumulated throughput of the waste (ore fines and charcoal) in the form in which they were donated to produce self-reducing briquettes. For both the fines of iron ore and of charcoal, it can be seen that they are predominantly composed of fine particles. Approximately 50% (D50) of the charcoal fines particles have sizes below 0.50 mm and the iron ore fines 0.20 mm, but 90% (D90) of the particles for both samples have sizes below 2.00 mm.
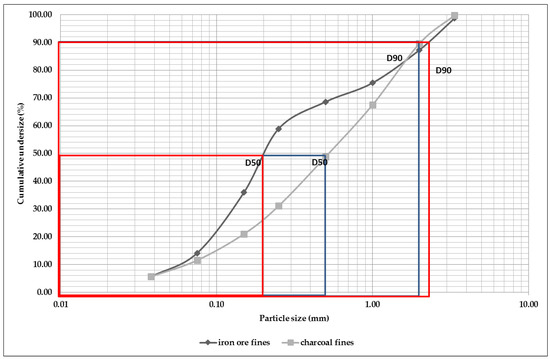
Figure 4. Particle size distribution curve of iron ore and charcoal fines.
However, in general, the curve of the charcoal fines has fewer fines compared to the iron ore fines. It was expected that the ultrafine and fine particles of the silica fume together with the fine particles of the charcoal and the iron ore would agglomerate quite homogeneously so that the voids could be filled efficiently. As finer particles are preferred for briquetting, pressure agglomeration is a prospective alternative process to agglomerate fines.
The results of the elemental analyses of the silica fume, iron ore fines and charcoal chips are described in Table 5, which shows the average values of the chemical analyses of the waste used to produce the self-reducing briquettes.
Table 5. Mean values of chemical analysis and humidity of the samples of silica fume, charcoal ashes, and iron ore fines.
| Oxides (%) | Waste | ||
| Silica Fume | Charcoal Ashes | Iron Ore Fines | |
| SiO2 | 90 | 65.86 | 38.46 |
| Al2O3 | 0.16 | 10.1 | 0.32 |
| P2O5 | 0.14 | 0.46 | 0.05 |
| CaO | 0.38 | 6.99 | 0.07 |
| TiO2 | 0.007 | 0.34 | 0.012 |
| MnO | 0.08 | 0.25 | 0.02 |
| Fe2O3 | 0.96 | 7.26 | 60.68 |
| MgO | 0.85 | 1.12 | - |
| Na2O | 0.5 | - | - |
| K2O | 2.83 | 2.45 | - |
| PPC | 4.78 | - | 0.39 |
| Moisture (%) | 1.86 | 2.13 | 1.29 |
As shown in Table 5, the silica fume presented a chemical composition typical of that found in ferrosilicon metallurgical industries. This is similar to the results described in several scientific works.It can, however, be noted that the K2O content is quite high. On the other hand, the chemical composition of the charcoal fines presented higher values of SiO2 than the chemical composition of the charcoal described in the literature. The iron ore fines have a chemical composition with a lower percentage of iron oxide than that the scientific literature stipulates is needed to produce FeSi, and contain high silica content compared to the published chemical composition of the iron ore. The analysis of the charcoal fines is presented in Table 6, which shows the percentage of moisture (water content of the material), ash (residual material after combustion), volatile materials (the content of material that is burned in the gaseous state) and fixed carbon (the content of material that is burned in the solid state). Based on the results described in Table 5, the components of the charcoal fines presented compatibility with similar analyses generated in the screening process of metallurgical and or steel mills. Compared to the charcoal bulk material, the ash content in the fines is much higher.
Table 6. Results of the immediate analysis of charcoal fines.
| Components | % |
| Volatile materials | 20.98 |
| Moisture | 2.13 |
| Ash | 26.88 |
| Fixed Carbon | 50.01 |
3.2. Apparent Density and Shatter Test in Briquettes
Figure 5a shows the self-reducing briquettes without binders (BM01). It is evident that these only obtained stipulated shatter resistance when the additions of water were 5.00%, 10.00% and 15.00% (R > 90%). This confirms that the greater the surface area and the lower the packing density, the greater the water demand will be. It is also important to note the effect that the amount of water exerted on the mechanical strength of self-reducing briquettes, even in those without binder addition. The added water possesses, due to its capillarity effect, the tendency to agglomerate the raw materials and confers initial resistance essential to its modelling.
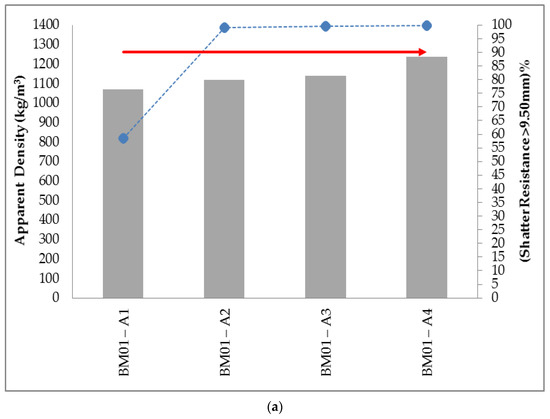
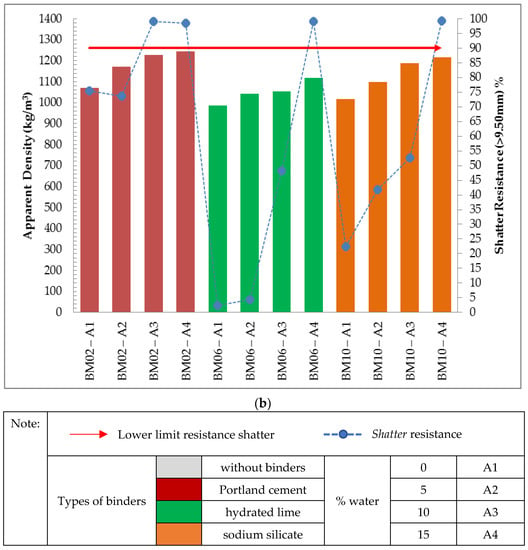
Figure 5. Relationship between the values of apparent density and shatter resistance obtained in 0.30 m drop for the self-reducing briquettes in composition (a) no binders (b) 2.50% binders.
Figure 5b shows the relationship between the apparent density and strength of briquettes with binders in the proportions of 2.50% for different types of binders, Portland cement, sodium silicate and hydrated lime. As presented, the apparent density for all treatments with a proportion of 2.50% binders obtained apparent density values within the stipulated range (950.00 kg/m3–1250.00 kg/m3). It was also found that the results obtained for the drop strength (0.30 m) were increasing in relation to the apparent density values in most treatments.
However, it was observed that the treatments with Portland cement binder obtained the highest values of apparent density (1070.97 kg/m3; 1170.08 kg/m3; 1226.68 kg/m3 and 1245.38 kg/m3) and minor variations for the results obtained for shatter resistance (0.30 m), although only two treatments (BM02-A3 and BM02-A4) obtained the shatter resistance index above the stipulated value (R > 90%).
On the other hand, the treatments with hydrated lime binder obtained the lowest values of apparent density (985.64 kg/m3; 1041.48 kg/m3; 1054.79 kg/m3; 1118.22 kg/m3) and greater variability for the obtained results of shatter resistance (0.30m), in which only 1 treatment (BM06-A4) obtained a value for the shatter resistance index above the stipulated R = 99.18%.
The treatments with sodium silicate obtained apparent density results between the values obtained (1017.01 kg/m3; 1099.68 kg/m3; 1187.05 kg/m3; 1217.47kg/m3) It is noteworthy that the highest obtained value of shatter resistance (0.30 m) comprised the treatment BM10-A4, R = 99.27%.
Figure 6 shows the relationship between the apparent density and strength of briquettes with binders at proportions of 5.00% for the different types of binders, Portland cement, sodium silicate and hydrated lime. The apparent density for all the treatments with binder proportion 5.00% obtained values within the stipulated density range (950.00–1250.00 kg/m3). Consequently, the results obtained for the shatter resistance (0.30 m) were increasing in relation to the apparent density values. It was noticed that all treatments obtained very close values of apparent density; with Portland cement binder (1059.32 kg/m3; 1113.40 kg/m3; 1128.86 kg/m3 and 1188.45 kg/m3), with hydrated lime binder (1013.66 kg/m3; 1028.08 kg/m3; 1074.3 kg/m3 and 1146.96 kg/m3), and with sodium silicate binder (1059.76 kg/m3; 1072.66 kg/m3; 1120.01 kg/m3 and 1165.39 kg/m3). In general, only two treatments with Portland cement binder (BM03-A3 and BM03-A4); one treatment with hydrated lime binder (BM07-A4) and two treatments with sodium silicate binder (BM011-A3 and BM11-A4) obtained the shatter resistance index above the stipulated value (R > 90%).
Figure 6. Relationship between the values of apparent density and shatter resistance obtained in 0.30 m drop for the self-reducing briquettes in composition 5.00% binders.
Figure 7 shows the relationship between apparent density and briquette strength with binders in the proportions of 7.50% for different types of binders, Portland cement, sodium silicate and hydrated lime. The treatments of self-reducing briquettes made with the three types of binders, with proportion of 7.50%, also obtained apparent density within the stipulated range (950.00 kg/m3–1250.00 kg/m3). However, regarding the shatter resistance, only two treatments made with Portland cement binder (BM04-A3 and BM04-A4), one treatment with hydrated lime binder (BM08-A4) and two treatments with sodium silicate binder (BM012-A3 and BM12-A4) obtained the shatter resistance index above the stipulated value (R > 90%).
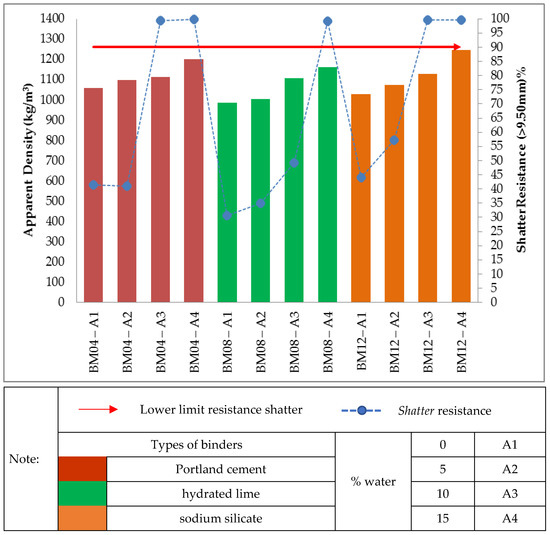
Figure 7. Relationship between the values of apparent density and shatter resistance obtained in 0.30 m drop for the self-reducing briquettes in composition 7.50% binders.
Finally, Figure 8 shows that the treatments of self-reducing briquettes with the three types of binders with a proportion of 10.00% also obtained apparent density within the stipulated range (950.00–1250.00 kg/m3). On the other hand, regarding the shatter resistance only two treatments made with Portland cement binder (BM05-A3 and BM05-A4), one treatment with hydrated lime binder (BM09-A4) and three treatments with sodium silicate binder (BM012-A2; BM012-A3 and BM12-A4) obtained a shatter resistance index above the stipulated value (R > 90%).
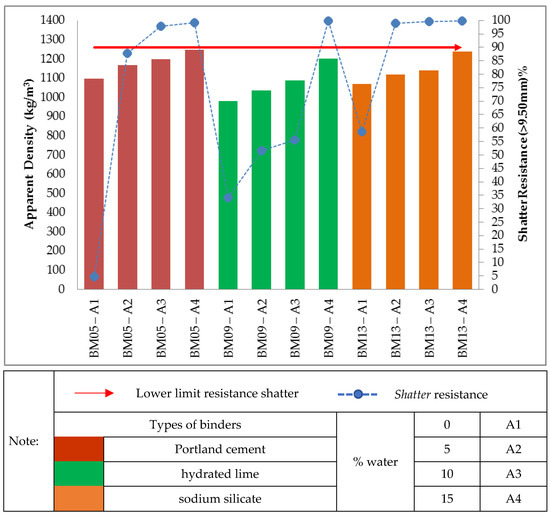
Figure 8. Relationship between the values of apparent density and shatter resistance obtained in 0.30 m drop for the self-reducing briquettes in composition 10.00% binders.
Thus, as shown in Figure 5, Figure 6, Figure 7 and Figure 8, of the 52 treatments of self-reducing briquettes tested for impact strength in a 0.30 m drop only 23 reached R > 90%.
The high impact strength of self-reducing briquettes using Portland cement as a binder was confirmed. According to the literature, when water is mixed with cement, various compounds are formed, including calcium silicate hydrate (C-S-H) and calcium hydroxide (C-H). In addition, by adding silica fume along with Portland cement, a reaction with (C-H) occurs, producing more (C-S-H), further increasing the mechanical strength of the mixture, and the ratio for inserting silica fume into cement mixtures is 15% to 20%. However, it is uncertain whether this can occur at these low temperatures.
The present work also explored the production of self-reducing briquettes using silica as the main component and the potential use of Portland cement as a binder. Furthermore, as certain variations in the mass of silica fume, binder and water proportions have already been investigated, an attempt was made to evaluate the variations in the mechanical strength of the mixture in accordance with the performed research.
In contrast, it is notable that the self-reducing briquettes made with hydrated lime binder (BM06; BM07; BM08 and BM09) only obtained the stipulated impact strength >90.00% when the maximum proportion of water, 15.00%, was used. Smaller proportions of water (0.00%; 5.00% and 10.00%) obtained unsatisfactory results, since the degradation of the materials was greater than 10%. These results were already expected, considering that mixtures containing hydrated lime as a binder already presented low strength and fragility, even during compaction.
Previous research produced briquettes from coal fines and different types of organic, inorganic, and combined binders; the following basic mixture (% dry matter) was assumed: coal fines-90, bio-mass-6 and binder-4. Thus, when using virgin lime or hydrated lime, the briquettes prepared with this type of binder did not show satisfactory results, causing deterioration both in compaction and later showing mechanical strength well below the assumed minimum (R = 85%). Regarding the study of [44], when investigating lignite agglomeration, they found that the cohesive properties of lime are not strong for some types of materials and that the proportion of lime should be between 25–30% when used as a single binder.
To conclude: when analysing the performance of the briquettes made with sodium silicate binder, they showed adequate mechanical strength for the following treatments: BM10—2.50% binder and 15.00% water; BM11—5.00% binder and when added 10.00% and 15.00% water; BM12—7.50% and when added 10.00% and 15.00% water; and BM13—10.00% binder and when added 5.00%, 10.00% and 15.00% water.
This is in accordance with the literature that described the mechanisms of briquetting in rotary kilns (RHF). In the cited work, the briquettes were produced with blast furnace dust and used sodium silicate as a binder. The results show that the oxygen and silicon bonds produced between the acidic silica gel particles from the curing reaction influence the negative ion connection bridge, linking the gel partitions and the sodium carbonate particles into a complex network structure.
The presence of liquids as free moisture between the particles, especially in a wet agglomeration process, causes cohesive forces between the particles, as the thin adsorption layers (≥3.00 nm thick) are immobile. They can form strong bonds between adjacent particles, smoothing the surface roughness and increasing the contact area between the partitions or decreasing the distance between the particles and allowing intermolecular attraction forces to participate in the bonding mechanism.
It is noteworthy that for all briquettes, the increase in apparent density of the briquettes resulted in gains in shatter resistance, generating an inversely proportional amount in mass of fines (Figure 9). Evidently, it is because higher densities provide a better packing and a more effective contact between the particles, reducing the empty spaces, which increases the resistance of the agglomerates.
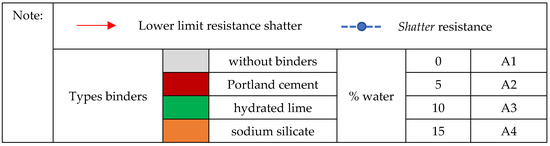
Figure 9. Relationship between the values of apparent density and shatter resistance obtained in 1.50 m drop for self-reducing briquettes with different types of binders and their respective proportions.
As the proportion of silica fume in the mixture increased, the tendency is a decrease in apparent density followed also by a decrease in the resistance of the briquettes. In relation to the different water additions, humidity provided a cohesive force necessary for the adherence of the particles to be agglomerated. This force also depends on the capacity of water adsorption by the particles, thus helping in the mechanical resistance. As shown in Figure 9, of the 23 treatments of self-reducing briquettes tested for impact strength in a 1.50 m drop, only 12 reached R > 90%.
3.3. Porosity in Self-Reducing Briquettes
The average porosity values are presented in Figure 10, respectively the twelve treatments of self-reducing briquettes tested for porosity determination. Of the twelve treatments of self-reducing briquettes that were analysed in the porosity determination tests, only ten met the premise of adequate porosity (45.00–55.00%), a parameter established to ensure that carbon monoxide formed in the furnace has access to the components of the pellets.
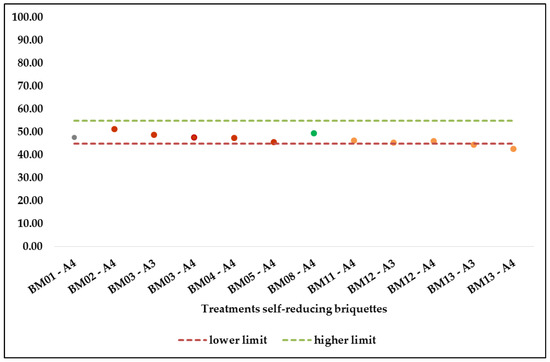
Figure 10. Mean values of the porosity results of the self-reducing briquettes.
It was observed in the self-reducing briquettes produced with Portland cement that, when there was an increase in the percentage of binder, a decrease in porosity resulted. Thus, it was found that the porosity of the briquettes depends on both the water/binder ratio and the degree of hydration of the mixture. For the results in the case of cement content, the H/C and S/C ratios increase in C-S-H, a component that promotes greater strength to the mixture. Furthermore, the increase in moisture allows a greater formation of Ca(OH)2, which results in a decrease of C in the C-S-H gel, therefore resulting in an increase in the S/C ratio and promoting the formation of a more compact composite with reduced porosity.
Briquettes produced with sodium silicate binder also showed a de-creasing value of porosity in relation to the increase in the percentage of binder. This shows that the gel particles penetrate into the briquettes, block the capillary pores, and the connection between the component particles of the briquette is reinforced by the silica acid gel particles. Therefore, increasing the binder content will decrease the number and size of the pores.
Furthermore, the only self-reduced briquette treatment produced with water satisfied the stipulated porosity premise. The same was true for the only treatment produced with hydrated lime. This corroborates the important relationship between the composition of water and binders.
After analysis and evaluation of the porosities of the self-reducing briquettes, the treatments that met the values within the stipulated range were forwarded for disintegration during thermal heating.
3.4. Disintegration during Thermal Heating
Figure 11 shows the average values of three repetitions of the resistance against thermal heating in relation to the temperature levels of 300 °C, 600 °C, 900 °C and 1200 °C. The goal for the thermal heating was to obtain a Rdr > 80%.
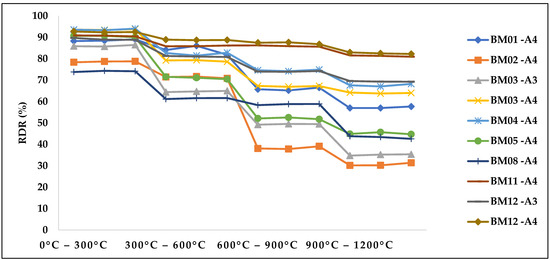
Figure 11. Influence of temperature on the resistance of self-reducing briquettes.
The resistance to thermal degradation and determination of the value of Rdr = 80% for the briquettes produced with the composition of waste electrofilter were also evaluated in the literature and their average value of Rdr was equal to 82.50%. These briquettes were produced with 23.00–27.00% silica fume, 51.00–53.00% carbon reducing agent, 4.00–5.00% silicon fines and 14.00–15.00% sodium silicate binder. In relation to the self-reducing briquettes of this present work, it was noticed that from 600 °C the resistance of some treatments of self-reducing briquettes produced with Portland cement reduced drastically for the studied levels. It is noteworthy that in the production of briquettes with Portland cement, none obtained Rdr > 80%. This is in accordance with study, in which it was found that the loss of strength related to the combined effect of the destruction of the binding phase (C-S-H) and the beginning of phase transformations of iron oxides. Sequentially, the treatment BM 08-A4 with addition of hydrated lime as binder showed resistance to thermal degradation with Rdr less than 45.00%, confirming published research.
It was noted that for the briquette samples without binders (from 600 °C), the Rdr was <80% and was not stipulated to meet the premise of resistance to thermal heating. However, in relation to the briquette samples without binders, BM01-A4, their resistance behaviour against thermal heating (Rdr = 57.65%) was higher than some of the samples that had Portland cement as binder (BM02-A4; BM03-A3 and BM05-A4).
The self-reducing briquettes with sodium silicate binder showed satisfactory results in which two samples (BM11-A4 and BM12-A4) met the premise established for defined selection, as demonstrated in Figure 11. The connections between sodium silicate and the constituent particles occur approximately at 1000 °C, thus at a higher temperature than the other agglomerants studied. This is in accordance with the analysis of the influence of sodium silicate in pellets of chromite and carbon fines, in which the binder added in briquettes of 4.00% with room curing presented the best performance even after exposure to high temperatures of 900 °C (1173K) to 1100 °C (1373K).
Finally, the hot mechanical resistance decreased at each elevation of the stipulated temperature levels and the respective exposure time to the thermal gradient. This occurrence is related to the breakage of bonds of binders and particles of the self-reducing briquettes, in addition to the output of the volatiles of the fine charcoal because in this atmosphere (air), part of the coal goes into combustion, which would not occur in an industrial oven. It was observed that the variations of types and proportions of binders, the proportion of water and composition of self-reducing briquettes are important factors to establish the pattern for the structural stability of self-reducing briquettes.
After performing the tests of resistance to degradation against thermal gradient, only two samples (BM11-A4 and BM12-A4) met the stipulated premise (Rdr > 80%). Figure 12 shows the images of the samples produced with sodium silicate binder (B12-A4; BM12-A3 and BM11-A4) after being submitted to the temperatures proposed for the thermal degradation tests.
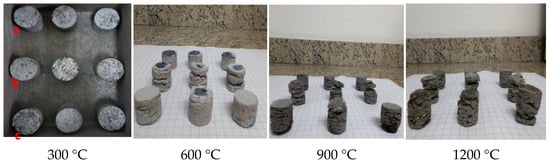
Figure 12. Self-reducing briquettes made with sodium silicate binder (a) BM-12-A4; (b) BM12-A3 and (c) BM11-A4 submitted to the degradation tests against thermal gradient.




