Study of the Carbothermal Reduction of Self-Reducing Briquettes Developed with Iron Ore Fines, Charcoal and Silica Fume Residues (3)
3.5. Analysis of the Crystalline Phases
The samples (BM11-A4 and BM12-A4) that obtained the value of Rdr > 80% were subjected to qualitative analyses by X-ray diffraction (XRD) and the main crystalline phases present in the raw materials of the self-educated briquettes were identified.
The results obtained by the analyses were consistent with others already reported in the scientific literature, related to iron ore fines, charcoal fines and silica fume.
As shown in Figure 13 and Figure 14 the presence of quartz (SiO2) phases was observed, this predominantly amorphous phase coming from silica fines and charcoal. Hematite phases were also identified relating to iron ore fines, one of the main representatives of iron minerals (Fe2O3) and in higher concentration in Brazilian mineral deposits. On the other hand, the presence of calcite (CaO) phases is predominantly related to charcoal fines.
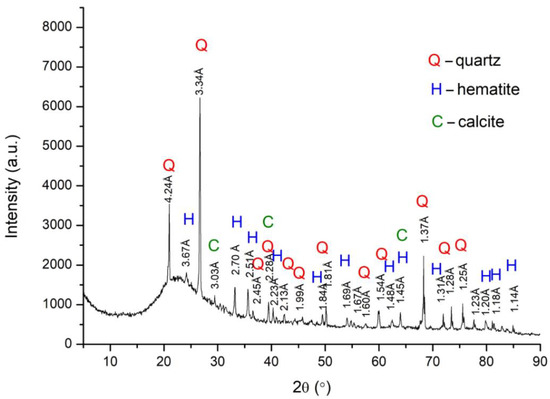
Figure 13. Analysis of the crystalline phases present in raw material of BM11-A4 self-reducing briquettes.
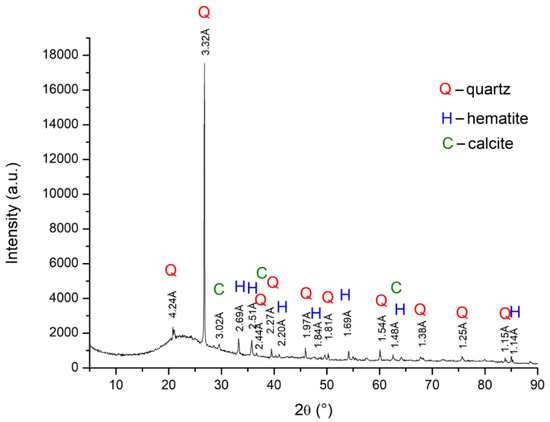
Figure 14. Analysis of the crystalline phases present in raw material of BM12-A4 self-reducing briquettes.
This indicates that the mineral phases that were found are oxides and high temperature furnace processes can reduce these. It is worth noting that the results obtained by XRD analyses corroborate with the content obtained from the chemical analyses performed by ICP, described previously in Table 5.
3.6. Reduction Tests
The loss of mass of the briquettes was an indicator for carbothermal reactions consuming the raw material while generating SiO(g) and CO(g). This means that the increase in temperature promoted greater production of gases and thus led to loss of mass. Figure 15 shows the total mass loss of each treatment of self-reducing briquettes subjected to the reduction tests at high temperature.
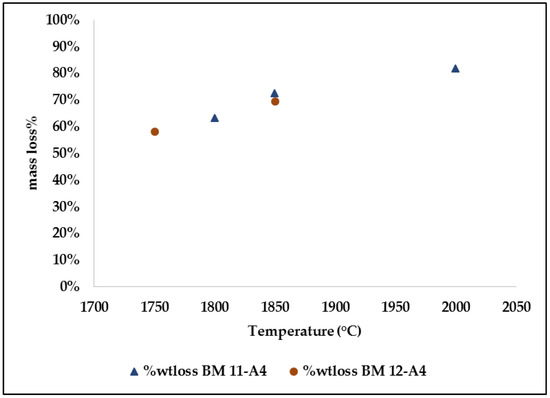
Figure 15. Influence of temperature on the mass of self-reducing briquettes.
The total mass loss was between 58.00 to 82.00% with temperatures increasing from 1750 °C to 2000 °C. Similar results to these were reported by other researchers using agglomerates of quartz fines and SiC; in the temperature range of 1550–1820 °C, the mass loss obtained was 49.3–85.5%. In the experimentation with agglomerates of quartz fines <70µm in reduction tests covering temperatures 1600 °C–1900 °C, it was respectively 55.8–77.7%. On the other hand, agglomerates produced with quartz fines of particle size 0.4 mm and SiC with particles of 0.5mm to 10.0mm, during heating at target temperatures between 1700 °C and 1900 °C, obtained mass loss of 35.61–90.13%. Thus, it was possible to verify that the results found are in consistency with the inherent mass loss of the raw materials during the processing and production of the metallic alloy. The cold load inside the SAF captures the SiO-gas. A sufficient reduction in the viscosity of the molten SiO2 at 1750 °C is observed together with a rapid loss of mass, possibly related to the increase of the reaction rate. The melting temperature of pure silica is 1720 °C, although softening and melting temperatures for different industrial quartz varieties vary between 1600 °C and 1800 °C.
Phase Distribution
At the temperatures of the reduction tests, all the metal was in the form of a Fe-Si liquid solution and when cooled after the experiments, it solidified into FeSi-FeSi2 or FeSi2-Si, depending on the amount of Si in the liquid solution. Thus, by checking how much Si, FeSi and FeSi2 that occurs in metallic phase, one can determine the composition of the metal. The metallographic analysis in SEM/EDS determined the composition of the individual phases present: metallic, carbonaceous and slag in the two treatments of self-reducing briquettes (BM11-A4 and BM12-A4) that were submitted to reduction. Confronting the results of mass or atomic composition of Si, of the metallic phases obtained by the EDS analyses, it is possible to relate them to the information provided by the Fe-Si diagram.
In accordance with the Fe-Si phase diagram, any Fe-Si alloy must contain more than 58.2% by mass of silicon to form silicon crystals during equilibrium solidification above the eutectic temperature.
The investigation of the original phases (SiO2, FeOx, C) as well as of the formed phases is of extreme importance for determination of the viability of insertion of the self-reducing briquettes as complementary load for the SAF. The formed phases will be SiC, FeSi with variable composition of Fe to Si, and the generation of slag of FeO, SiO2 and oxides originating from the agglomerate. SiC and FeSi are interesting components in a self-reducing briquette.
Figure 16 shows cross-section views of a sheet containing metallic Si and FeSi2 phases and formation of the carbonaceous SiC phase after reaction at 1800 °C for 30 min of the BM11-A4 treatment. Along the whole SiC/graphite interface, metallic particles of micron size were found. It is noteworthy that, as shown in Figure 16a,b there was a prevalence of SiC phase formation in all visible phases. Figure 16c shows metallic incrustations and slag. Figure 16d shows the condensation of SiO gas, at 1800 °C, at the graphite/SiC interface on the top of the crucible. SiO-gas is one of the reaction products of the silica at high temperature. It was observed that the SiO gas from the self-reducing briquettes reacted with the C(s) in the crucible and produced some carbonaceous phase granules of SiC(s) embedded in the crucible. This indicates that some of the silicon left the experimental system as SiO gas; as shown in Equations (6)–(8):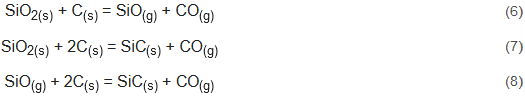
Figure 16. Scanning electron microscopy (SEM) image sample of the BM11-A4 treatment that reacted at 1800 °C for 30 min. Slag, SiC and metal phase samples, respectively, indicated by red arrows: (a) metal phase and SiC interface; (b) predominant SiC; (c) metal phases interface with slag; (d) graphite/SiC interface (top of crucible).
Slag formation containing predominantly CaO-SiO2 (32.55–76.27%) was also observed, the optimum values in slag composition for silicon production being 75% in the metal phase, with composition 29.1 % Al2O3, 25.9 % CaO and 45 % SiO2.
Figure 17 shows a cross-section of the BM11-A4 treatment under a temperature of 1850 °C for 30 min. The amount of carbon present in the FeSi2 phases is smaller in comparison to the chemical composition at a temperature of 1800 °C, which suggests that the increase in temperature favours the carbo-chemical reactions inherent to the FeSi production process, with the gradual evolution of the C(s) consumption reactions (7, 16, 55). Two mechanisms of Si production have been observed: transpiration of Si metal from the condensate layer around the crucible and production of Si in SiC particles. Figure 17a,b, shows the presence of metal droplets containing Si phases, FeSi2 and those containing SiC incrustations. Figure 17c shows that there was formation of the metallic phase of FeSi2, but also SiC particles containing high contents of SiO2, CaO-Al2O3. Although the FeSi production process is considered to be a slag-free process due to the high purity of the raw materials, there is still slag formation. The most abundant oxide impurities are the aluminium (Al2O3) and calcium (CaO) oxides that form a phase together with SiO2, to form an Al2O3-CaO-SiO2 system. Figure 17d shows the image of the crucible bottom containing slag and SiC, with demonstration of the metallic pockets formed on the SiC and slag/graphite interface.
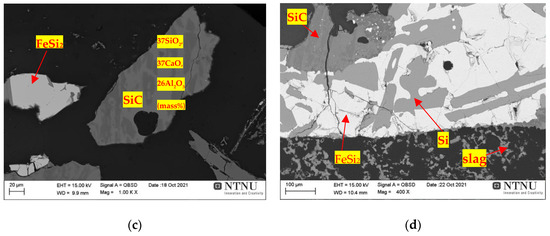
Figure 17. Scanning electron microscopy (SEM) image sample of the BM11-A4 treatment that reacted at 1850 °C for 30 min. Slag, SiC and metal phase samples, respectively, highlighted by red arrows: (a,b) metal phase/SiC interface (c) metal phases and SiC/slag interface; (d) deposition of metal phases on SiC and slag/graphite interface (crucible bottom).
Figure 18 shows a cross-section of the BM11-A4 treatment at 2000 °C for 30 min, in which metallic and SiC phases were found. Especially at 2000 °C, the formation of metallic droplets with phase composition in the FeSi2-FeSi area can be observed (Figure 18a,b) which demonstrates that metal formation occurred below FeSi 50% [59]. The presence of SiC(s) presented a lower proportion in the Si/SiC ratio, having a less porous and more consolidated characteristic, besides having higher carbon contents (%) than at the temperatures previously investigated. At 2000 °C the SiC particle is much denser, and the original carbon structure no longer exists in the samples. SiC was found on the surface of FeSi heated at 2000 °C; however, it presented in lower proportions in the Si/SiC ratio than at the lower temperatures (1800 and 1850 °C) investigated previously. The reason for this is not fully understood, but one explanation may be that SiC is formed on the silicon surface particles/droplets at low temperatures due to the presence of small amounts of CO and that SiC reacts with silica at higher temperature. What is suggested is that the increase in temperature led to the carbo-chemical reactions inherent to the FeSi production process, with the gradual evolution of the C(s) consumption reactions.
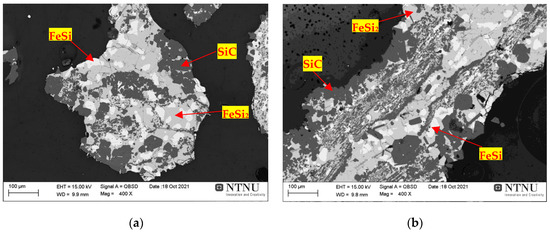
Figure 18. Scanning electron microscopy (SEM) image sample of the BM11-A4 treatment that reacted at 2000 °C for 30 min. Slag, SiC and metal phase samples, respectively, highlighted by red arrows: (a,b) metal phase/SiC interface.
Figure 19 shows the products obtained after reduction of treatment BM12-A4 It is worth noting that there was formation of the FeSi metallic phase at 1750 °C for the BM12-A4 treatment (Figure 19a,b), which was not observed at 1800° for the BM11-A4 treatment (Figure 16).
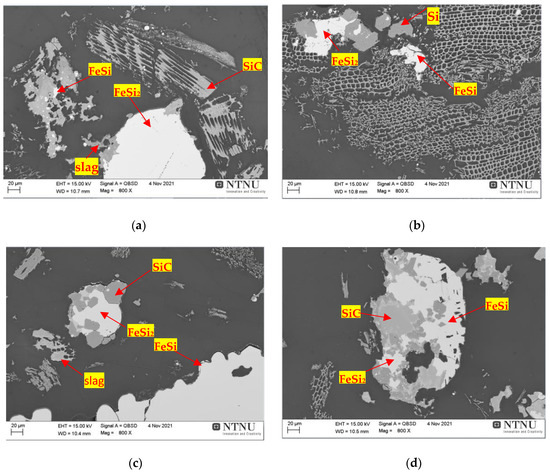
Figure 19. Scanning electron microscopy (SEM) of the imaging sample of BM12-A4. Slag, SiC and metal phase samples, respectively, highlighted by red arrows: (a,b) metal phase/SiC interface treatment that reacted at 1750 °C for 30 min; (c,d) metal phases and SiC/slag interface treatment that reacted at 1850 °C for 30 min.
Comparing the treatment BM12-A4 (Figure 19c,d) with the phases of the treatment BM11-A4 (Figure 17), both at a temperature of 1850 °C, the phases produced were similar. In fact, when comparing the results obtained for the FeSi and FeSi2 phases, there were no strong variations in the Si, C and Fe contents. However, on the other hand, it was observed that in the slag produced in both treatments the percentage of CaO present was 30.18% for BM 11-A4 and 5.05% for the BM12-A4 treatment, which may be related to the lower proportion of 2.50% of active silica in the BM12-A4 treatment.
It is worth noting that the values obtained by calculating the equation of reactions and the mass balance applied for this research do not seem to fit the 75% FeSi production, due to the larger amount of SiC that can be seen in the micrographs generated by SEM. Thus, when the calculations are based on a Si content of 60% in the alloy to produce 75% FeSi, the theoretical amount of SiC produced would be around 1.90 g for treatment BM11-A4 and 2.70 g for treatment BM12-A4 respectively a percentage of 7.60 and 10.72% for the total mass of the treatments studied. Thus, if the average Si in the metal was assumed to be 50%, the percentage values would better represent the results of the products obtained. Thus, the theoretical amount of SiC produced would be around 4.10 g for treatment BM11-A4 and 4.90g for treatment BM12-A4, respectively; a percentage of 16.40% and 19.60% for the total mass of the researched treatments.
Finally, it is assumed that this occurrence is due to the heterogeneity of the raw materials, which are wastes, which may also describe the deviation, i.e., the actual analyses would not be the average analyses assumed for the self-reducing briquettes produced.
4. Conclusions
It was concluded that the thirteen types of mixtures studied presented adequate briquetability, but the variations of binder and water percentage affected structural behaviour and process operability. There was an increase in the apparent density in relation to the elevation of the water/binder proportion of the mixture for all the self-reducing briquettes produced. It was also noted that with the increase in the proportion of binders—Portland cement and sodium silicate—there was a decrease in the porosity. In relation, the resistance to hot thermal degradation was decreasing with each increase in the temperature/time of exposure to thermal gradient, with this characteristic being observed in all types of self-reducing briquettes submitted to these tests. Consequently, only two of the produced treatments BM11-A4 (5.00%) and BM12-A4 (7.50%), both with addition of sodium silicate binder, showed Rdr > 80%.
As for the reduction tests, it was shown that the steps of the FeSi process can be simulated in a small-scale induction furnace, even using only self-reducing briquettes. The formation of metallic Si particles was evident from 1750 °C onwards. At temperatures from 1800 °C to 1850 °C, the analysed metal had 50/50 Si-FeSi2 phases, i.e., FeSi75. However, some metal nodules are found in the area with FeSi-FeSi2 phases, below 50% FeSi, especially at 2000 °C. In addition, it was observed that Si was also condensed as SiO gas, which can be seen at the top of the carbon crucible, and this was partially transformed into SiC. In addition, no SiO2 was found except as CaO-Al2O3-MgO-SiO2 slag.
Additionally, the relative percentage error with respect to the introduction of silicates and the carbonaceous material into these agglomerates was calculated. Thus, describing a relative error of 4.26% for the BM11-A4 treatment and 4.50% for BM12-A4 for the silica fumes, the results were present as if there was a smaller amount than reported. On the other hand, the fines for the charcoal showed results as if there was a greater amount of carbonaceous material than reported in both treatments, showing a relative error of 5.88%.
Thus, it is understood that the introduction of self-reducing briquettes as a possible complementary load could represent a viable and advantageous alternative, being used in the production of FeSi types that have wider specification limits.
This work added significant and unpublished data, which can contribute positively in relation to the properties presented by self-reducing briquettes with the respective wastes from the FeSi industrial segment, but pointing out the need for further research in order to optimize its metallurgical processing, aiming at sustainable development that is a necessary commitment to the industrial sectors and that is certainly correlated with the ore extraction enterprise and its metallurgical processing.
Pascoal, A. D., Rossoni, H. A., Kaffash, H., Tangstad, M., & Henriques, A. B. Study of the Physical Behaviour and the Carbothermal Reduction of Self-Reducing Briquettes Developed with Iron Ore Fines, Charcoal and Silica Fume Residues. Sustainability, 14(17), 10963. https://doi.org/10.3390/su141710963




