A Study on the Influence of Drying and Preheating Parameters on the Roasting Properties of Limonite Pellets (1)
1. Introduction
Iron ore pellets as a high-quality charge possess excellent metallurgical properties and offer advantages such as the improved permeability of the blast furnace charge column. Iron ore pellet drying, preheating, and roasting are important parts of the pellet ore production process; improving pellet drying efficiency and pellet strength is related to the improvement of the quality of the pellet minerals. To produce high-quality oxidized pellets, a reasonable selection of process parameters is essential.
Limonite is characterized by its low content of harmful elements and is a low-grade iron ore. However, low-grade ores often contain high levels of impurities, which can lead to low pellet strength when using limonite. In addition, the pelletized ore undergoes a number of handling, transport, stacking, and movement processes before and after entering the blast furnace. These processes are subject to a variety of severe mechanical forces such as collision, impact, compression, and friction. These mechanical forces can cause some pellets to break up and produce fines, which can affect furnace operation and production targets. In order to solve these problems, it is possible to increase the density and mechanical strength of the pellets by adding binders to fill the gaps and cracks between the ore particles and bond them together. In the steel industry, binders are essential for the pelletization of iron ore concentrates. Bentonite has strong water adsorption capacity, high bonding strength, and a relatively low market price, and is the main binder used in the preparation of pellets. Bentonite inclusions in the impurity content are generally high; every 1% increase in bentonite in the proportion of raw materials will lead to a reduction of about 0.4% to 0.6% of the iron grade of the pellet ore and affect many of its important performance indicators. In particular, it increases the slag load in blast furnace smelting, thus reducing the blast furnace utilization factor. At the same time, the addition of too much bentonite will increase the slagging and coking tendency of the furnace; this coking and slagging material will be attached to the furnace wall or furnace cylinder, and the smelting effect and the blast furnace life will be adversely affected. Compared with foreign countries, the amount of bentonite in domestic pellet plants is higher, generally 2.0% to 3.5% or even higher, with only a few plants having levels below 2.0%. Therefore, the discussion of by how much to reduce the amount of bentonite and improve the grade of limonite ore pellets has become the current focus of many scientific research institutes and plant research directions.
Pellet ore drying, preheating, and roasting are important parts of the pellet ore production process; improving the pellet drying efficiency and pellet strength is related to the improvement of the quality of pellet minerals. Prior to the roasting of the pellets, it is necessary to preheat them. This is because there is still a large amount of crystalline water inside the pellet; if direct roasting leads to rapid evaporation of the internal crystalline water, the pellet shell cannot withstand the phenomenon of cracking or blowing up. Also, the temperature and time of preheating will have an effect on the mechanical strength and properties of the final finished ball.
The results of many studys show that the preheating temperature affects the compressive strength and reducibility of the finished balls after roasting. Liu Kai and his team conducted an in-depth study of magnetite pellets using a roasting temperature of 1300 °C. They found that the crystal bridge linkages were further tightened with the increase in roasting time, which enhanced the compressive strength of the pellets. In the study, when the roasting time reached 20 min, it was observed that the distribution of Fe2O3 grains became more homogeneous, and the crystal bridge linkages were optimized. This means that the crystal structure of the magnetite pellets was fully adjusted and stabilized during the 20 min roasting time. The compressive strength of the pellets was successfully increased by enhancing the linkage of the crystal bridges. The recrystallization and grain growth processes within the pellet can be carried out smoothly within a suitable temperature range. Therefore, it is crucial to ensure proper temperature control during the roasting process of pellets.
Also, pellet diameter is a key parameter, and the effect of pellet diameter on the performance of pelletized ores involves further key parameters such as mass and heat transfer rates, diffusion rate, and reaction surface area. Therefore, when designing and operating a pelletizing process, the diameter of the pellets needs to be reasonably controlled to achieve the desired ore properties. Zhang Pan conducted a roasting process study on high-sulfur magnetite at a site, and some important conclusions were drawn by comparing the results of pellets with different particle sizes under natural ventilation and blast-roasting conditions. For the same pellet quality, the larger the grain size of the pellet, the higher the pass rate, which means that larger grain sizes are more likely to meet the desired quality standards during the roasting process. At the same time, the strength of the roasted pellets was also better, indicating that larger-grain-size pellets have better mechanical properties.
2. Materials and Methods
2.1. Test Material
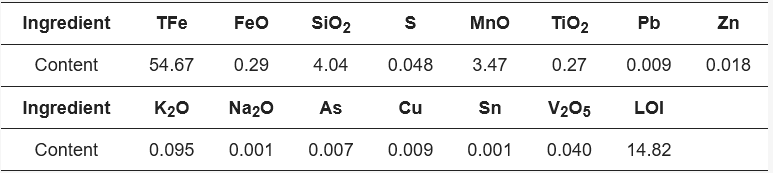
The sample material included in the experiment was Yunnan limonite iron ore, and the main chemical composition of limonite is shown in Table 1. The total iron content of limonite is 54.67%, with a relatively low grade. The veinstone composition is mainly SiO2, with a mass fraction of 4.04%, and contains more types of impurities, but the content is small, with a loss on ignition of 14.82%. Yunnan limonite is shown in XRD in Figure 1, where XRD analyses were carried out to determine the mineral composition of the raw material. As shown in Figure 1, the major mineral forms of limonite are FeO(OH) and Fe2O3.
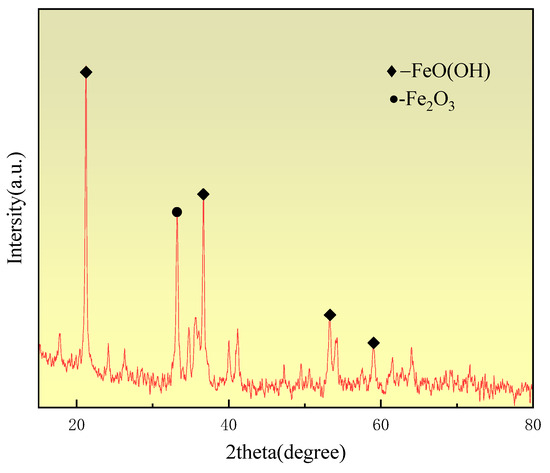
Figure 1. XRD pattern of limonite.
Table 1. Main chemical composition of limonite (/%).
The SEM images of limonite are shown in Figure 2. It is known that the limonite particles from plants in Yunnan become granular, with a rough surface and loose and porous organization. Strong water absorption, a shape similar to coral on the seabed, and the large wet capacity of the powder show the typical limonite crystallization state. This means that limonite has a strong surface hydrophilicity during the pelletizing process, forming more liquid film and tending to bond to more iron ore particles, which is favorable for the growth of pellets.

Figure 2. SEM maps of limonite: (a) 5000×, (b) 8000×, (c) 10,000×, (d) 15,000×.
Table 2 lists the chemical properties of bentonite: bentonite as a pellet binder actually plays a role in montmorillonite, gel price reflects the montmorillonite dispersion performance indicators, and the water absorption rate indicates the bentonite water-absorbing capacity of the indicators. The montmorillonite mass fraction of the bentonite used was as high as 85.97%, which indicated that the bentonite had good physical property indexes.
Table 2. Composition and properties of bentonite.
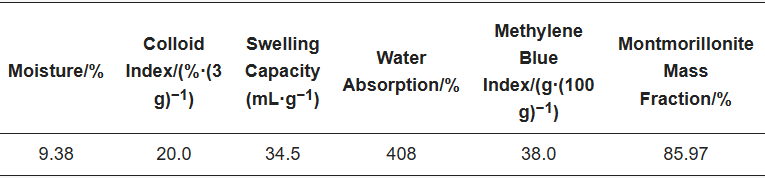
The composition and properties of the bentonite used in the experiment are shown in Table 2.
2.2. Test Methods:
(1) Pellet production: The experimental process is shown in Figure 3, including the grinding of limonite powder using a ball mill, screening, mixing of additives, molding of raw balls, and determination of strength properties of finished pellets. Before the experiment, a new type of ball mill was used to grind limonite ore from a plant in Yunnan for 72 h, a 100-mesh automatic sieving machine was used to screen the mineral powder for the experiment, 200 g of 100-mesh limonite iron ore powder for the experiment was mixed with bentonite clay according to the certain proportions of the ball in a disc ball-making machine, 3% of distilled water was added in equal amounts to the mineral powder for wetting, and 7% of distilled water was sprayed in order to make a pellet. After stopping the addition of water and material, the raw pellets continued to roll in the disc ball-making machine for about 2 min until the pellets were dense. They were then taken out with a spoon and then a vernier caliper was used to separate the pellets; the particles with diameters of 8–15 mm were left for spare parts, and the rest of the unqualified pellets were discarded. The pellets were dried in a desiccator at 110 °C for 1 h to remove moisture and volatiles for subsequent use.
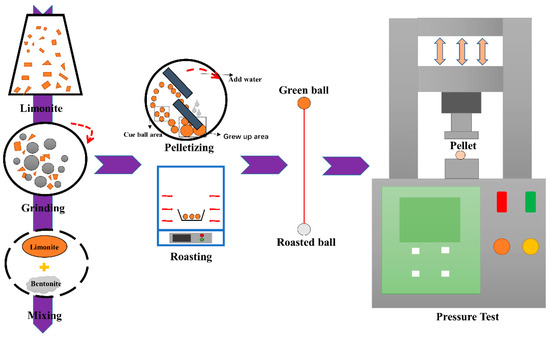
Figure 3. Limonite pellet preparation process.
(2) Pellet roasting: The raw pellets were transferred to the crucible in batches. The crucibles with the pellets were baked in a muffle furnace. The roasting temperature was set as follows, as shown in Figure 4: ramped up to 200 °C over 20 min and held at 200 °C for 20 min; ramped up to 700 °C over 20 min and held at 700 °C for 20 min; and increased to 1250 °C over 40 min and held at 1250 °C for 30 min. Subsequently, the pellets were gradually cooled to room temperature and removed from the furnace.
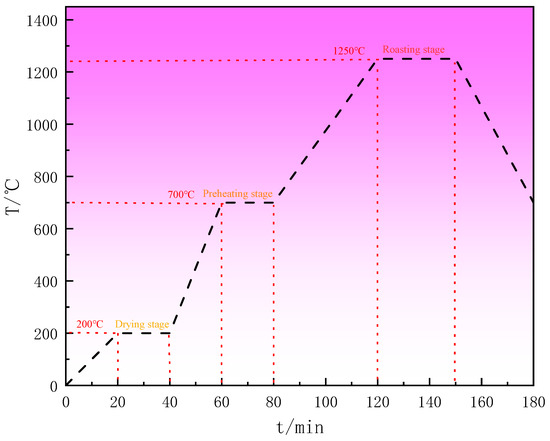
Figure 4. Stepwise heating temperature profile of limonite pellets.
(3) Measurement and testing: The diameter and weight of raw and roasted pellets were measured using an electronic balance. The compressive strength of raw pellets and roasted pellets was determined by using the raw pellet compressive strength tester DS2 and digital display pressure tester SKE-10KN. Using a vernier caliper to select pellets with a diameter of 8–15 mm, the average compressive strength of raw and roasted limonite pellets was recorded in accordance with YB/T 4848-2020 Physical Test Methods for Roasted Pellets.




