Developing Iron Ore Pellets Using Novel Binders for H2-Based Direct Reduction (2)
3. Results and Discussion
3.1. Sample Characterization
The chemical compositions of the magnetite iron ore concentrate and bentonite are given in Table 3. It is observed that the magnetite concentrate is of high grade with small impurities of magnesium oxide and silica, which may have resulted from the presence of the associated gangue of olivine mineral. For the phase composition of magnetite concentrate, Figure 1 depicts only the presence of magnetite as the main phase. The particle size distribution analysis of the magnetite ore concentrate shows that 98 wt.% of the magnetite ore concentrate particles were below 100 μm and 84.6 wt.% of the particles were under 50 μm.
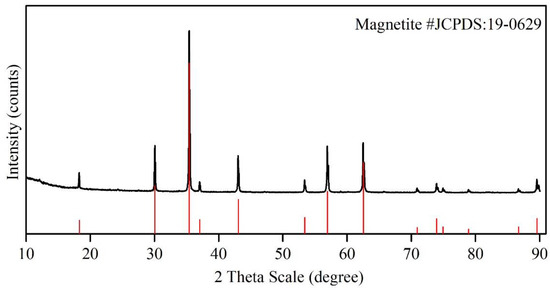
Figure 1. Phase composition of magnetite concentrate. Red: standard diffraction data of magnetite mineral according to JCPDS:19-0629; Black: diffrac-tion lines of the sample.
Table 3. Chemical composition of magnetite concentrate and bentonite.
| Materials | Chemical Composition, wt.% | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Fe | SiO2 | CaO | MgO | Al2O3 | K2O | Na2O | TiO2 | |
| Magnetite concentrate | 66.16 | 1.60 | 0.15 | 1.81 | 0.18 | 0.03 | 0.06 | 0.014 |
| Bentonite | 3.22 | 59.6 | 0.1 | 3.1 | 21.9 | 0.5 | 3.1 | 0.8 |
3.2. Moisture Content of Green and Dry Pellets
Figure 2 shows the moisture content of the green and dried pellets produced from all recipes. It is evident that the quantity of water needed for each recipe varies based on the quantity and type of binder used. It is worth mentioning that the amount of water required to activate the binder is dependent on its nature and composition. Recipes that included CMC (U1–U6) required a higher amount of water addition, resulting in the highest moisture content in green pellets, ranging from 12 to 18 wt.%. This can be attributed to the chemical composition of CMC, where it contains several hydroxyl groups that bond with several water molecules by hydrogen bonds. Moreover, the swelling/dissolution phenomenon is where CMC fibers require water to swell and dissolve, but the fibers swell first before they dissolve. The KemPel recipes (K1–K3) show a moisture content of 12–14 wt.%. Both Alcotac FE 16 (H1–H7) and Alcotac CS (C1–C8) show a moisture content between 8 and 11 wt.%. All dry recipes contain less than 0.5 wt.% moisture content after drying the pellets for 2 h at 105 °C.
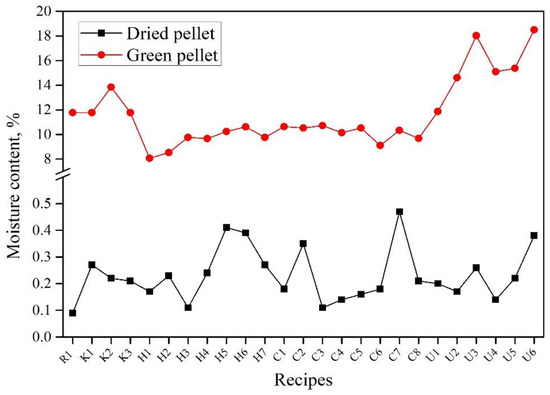
Figure 2. Moisture content of green and dried pellets of all recipes.
3.3. Pellet Size Distribution
The pellet size distribution of all recipes was measured to detect the yield of the desired size range (9–16 mm). Figure 3 shows the yield of all recipes divided into four groups according to the added organic binder. As the pelletizing conditions differ for each of the recipes, the yield also differs accordingly. Thus, while it is not entirely appropriate to compare the yield of different recipes, it is worth noting that in terms of ease of pelletizing, Alcotac FE 16 (H) showed the best performance, followed by Alcotac CS (C). The lowest yield resulted from using CMC binder (U), and this could be attributed to using excessive water, which causes wet pellets to cohere with each other and difficulties with it being discharged from the balling disc. As mentioned before, this result cannot be generalized as the pelletizing conditions and techniques differ from one recipe to the other.
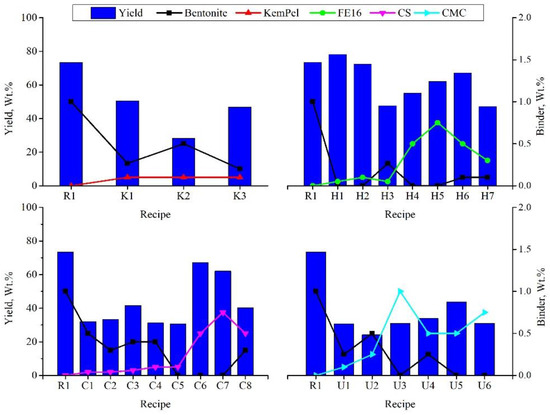
Figure 3. Variation in the yield of the desired pellet size for all recipes.
3.4. Drop Test Measurement of Dry Pellets
Ten over-dried pellets from the size range of 9–12.5 mm were subjected to the drop test, where the number of drops each pellet could withstand without breaking was counted. The final drop number value is the average drop number of 10 pellets. Figure 4 presents the drop test results for all recipes. The KemPel recipes (K) that represent partial replacement of bentonite showed good drop test results in comparison with the reference recipe, R1, while the drop test results of recipes with complete replacement of bentonite by Alcotac FE 16, Alcotac CS, and CMC are in order of CMC > Alcotac CS > Alcotac FE16.
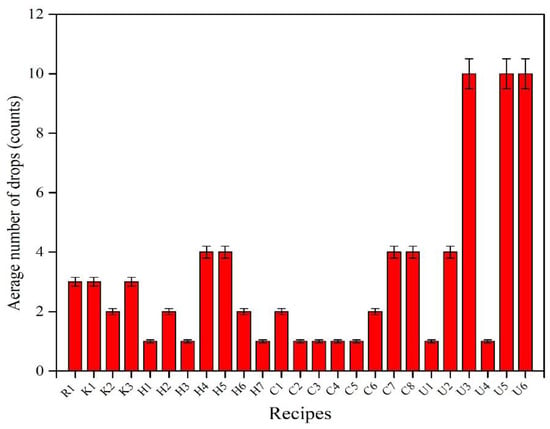
Figure 4. Drop test of dried pellets of all recipes.
In addition, the drop test of fired pellets was carried out using 10 pellets of each fired R1, K1, H4, C7, and U5 recipe. The test was performed from a height of 2 m onto a steel plate, and the number of drops they could withstand without breaking was noted. It was observed that all recipes could withstand 15 drops. Some of the pellets could also withstand more than 15 drops, but a limit of 15 was set for the measurement. Thus, the firing increases the drop number for all pellets drastically.
3.5. Cold Compression Strength (CCS) of Dry and Fired Pellets
The CCS of dry and fired pellets was measured for a total of 10 pellets with a diameter of 9–12.5 mm for all recipes. Figure 5 shows the variation in CCS of all produced recipes. It can be seen that recipe K1 in which 73.5 wt.% of bentonite in the R1 recipe was replaced with 0.1 wt.% of KemPel, showed comparable CCS to the reference recipe (R1), while the other two recipes (K2 and K3) had poor results when compared to K1 and the reference recipe. When using CMC, it can be observed that all the recipes except U1 had either comparable or better results than the reference recipe with bentonite. If we look at recipes U3, U5, and U6, where there is no bentonite at all, and the amount of CMC is increased from 0.5 to 1 wt.%, it can be seen that by increasing the amount of CMC, the CCS increases. With the addition of 1 wt.% CMC, the CCS increases significantly. A similar result can be seen in recipes H4 and H5 with Alcotac FE16. Neither H4 nor H5 contain any bentonite, and the amount of Alcotac FE 16 is 0.5 wt.% and 0.75 wt.%, respectively. As the amount of Alcotac FE 16 increases, the CCS also increases. On the other hand, the recipes that contain Alcotac CS show a lower CCS than the reference recipe. The recipes, C6, C7, and C8 were the recipes that demonstrated a better CCS compared to the other recipes with Alcotac CS. According to the CCS results of dried pellets, the best CCS recipes (R1, K1, H4, C7, and U5) were subjected to firing followed by CCS testing.
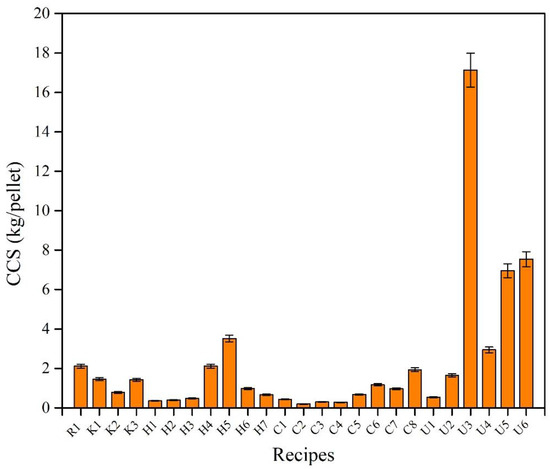
Figure 5. CCS of dried pellets of all recipes.
Figure 6 shows the CCS of the fired recipes R1, K1, H4, C7, and U5. Ten pellets each from the size ranges 9 to 12.5 mm were subjected to the CCS measurement, and an average was taken as the final value. It was noticed that the CCS values of all selected recipes were improved after firing. The reference recipe (R1) with bentonite displayed the highest value of CCS in comparison with other organic-bonded recipes. For organic-bonded recipes, the CCS values can be described in the order of K1 > C7 > H4 > U5. Although the K1 recipe saves 73.5% from used bentonite in R1, it still shows high CCS with only an 8% decrease compared to that of R1. By fully replacing bentonite with organic binders in C7 (0.75% Alcotac CS), H4 (0.5% Alcotac FE 16), and U5 (0.5% CMC), the CCS value decreased by 37.5%, 41.66%, and 75%, respectively, when compared to the reference recipe, R1. However, while the CMC recipe U5 showed the most promising dry CCS results compared to all the other recipes, even the reference recipe (R1), it shows the worst CCS after firing; this may be due to the complete decomposition of CMC at a low temperature, 390 °C.
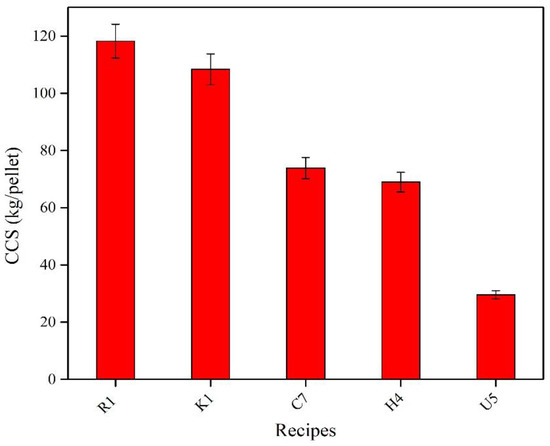
Figure 6. CCS of selected fired pellets.
3.6. Reducibility of Dry and Fired Pellets as well as CCS after Reduction
The reduction behaviors of selected recipes R1, K1, H4, C7, and U5 were tested using non-isothermal thermogravimetric analysis in H2 gas as a reducing atmosphere at temperatures up to 950 °C. This test was performed for the dried and fired recipes under the same conditions. Figure 7 depicts that the dried pellets, in general, show better reducibility than fired pellets. At any given time and temperature, the reduction extent is always higher for dried pellets compared to the fired pellets. During the non-isothermal heating stage, the reduction extent of dried pellets was in the order of U5 > C7 >H4 > K1 > R1, where a 55% reduction extent was achieved by organic-bonded pellets (U5, C7, and H4), and the lowest reduction extent, 35%, was displayed by the bentonite-bonded pellet (R1) and partially bentonite-bonded pellets (K1). During the isothermal stage at 950 °C, the same trend was observed where R1 and K1 pellets needed 41 min to approach the complete reduction, while U5, C7, and H4 needed approximately 19 min until complete reduction. This trend can be attributed to the porous structure resulting after the thermal decomposition of the organic binder, which facilities the H2 diffusion and enhances the reduction of the pellets. In contrast, the fired pellets showed an opposite order. During the non-isothermal stage, a 50% reduction was observed in the case of the bentonite-bonded pellet (R1), and the lowest value, 32%, was displayed by the Alcotac FE 16-bonded pellet (H4). Thus, the reduction extent order was as follows R1 > C7 > K1 > U5 > H4. Moreover, during the isothermal stage, at 950 °C the same trend was observed, where R1- and C7-fired pellets needed 37 min to reach the complete reduction, while K1, U5, and H4 needed 56 min until complete reduction. This behavior can be attributed to the effect of different parameters such as the gangue associated with magnetite ore and binder composition, for example, the presence of magnesium oxide gangue, which reacts with the iron oxide during the firing process of pellets and eventually forms islands of magnesioferrite (Mg2Fe2O4) surrounded by hematite [29,30]. When the reduction takes place at 800 °C, the temperature allows for the slow diffusion of magnesium from the magnesioferrite into the surrounding iron oxides. This diffusion is slightly more pronounced if hematite is reduced to wustite instead of magnetite. At 900 °C, the diffusion of magnesium was accelerated, leading to the disappearance of the boundaries of magnesioferrite islands. It was reported that the structure formed within the magnesioferrite phase during sintering remained unaffected by the reduction treatment up to 900 °C [30]. The presence of bentonite in the sintering process hinders the formation of magnesioferrite during sintering, thereby promoting the reduction of bentonite-bonded fired pellets. This demonstration was confirmed by XRD analysis of the reduction products of dried and fired R1 and H4 pellets, see Figure 8. It was observed that the phase compositions of the reduction products of the dried R1, dried H4, and fired R1 pellets revealed that the only detected phase is the metallic iron (Fe), while the reduced fired H4 shows the presence of a metallic iron phase with traces of a magnesium iron oxide phase (MgO0.77FeO0.23), which supports the formation of hardly reduced magnesium ferrite.
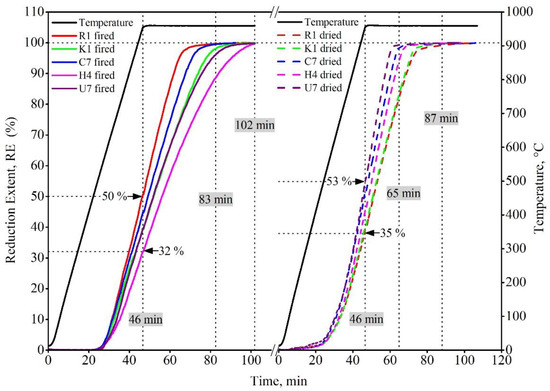
Figure 7. TGA of selected dried and fired pellets.
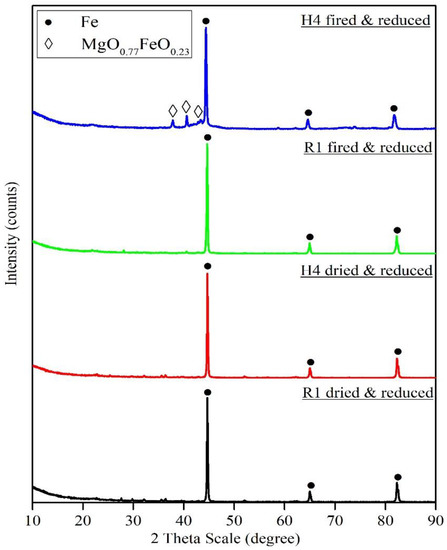
Figure 8. Powder XRD patterns of reduction products of dried and fired R1 and H4 recipes.
The CCS results for the reduced fired and dried pellets are given in Figure 9. For reduced fired pellets, it can be observed that all the organic- and bentonite-bonded pellets, except the CMC-bonded pellets, manifest low CCS values after reduction. For the CMC-bonded pellets (U5), there was no change in the CCS value after firing and reduction. However, the CCS value was still less compared to the other organic-bonded and reference pellets. The bentonite-bonded (R1), KemPel-bonded (K1), Alcotac FE 16-bonded (H4), and CMC-bonded (U5) pellets showed a similar CCS value after reduction, and it was approximately 30 kg/pellet. The Alcotac CS-bonded (C7) pellets showed even less strength, and it was approximately 15 kg/pellet. On the other hand, the dried pellets showed a higher strength after reduction. The highest increase in the CCS value was for the reference recipe (R1) followed by KemPel-bonded pellets (K1), Alcotac FE 16-bonded pellets (H4), Alcotac CS-bonded pellets (C7), and CMC-bonded pellets (U5), respectively.
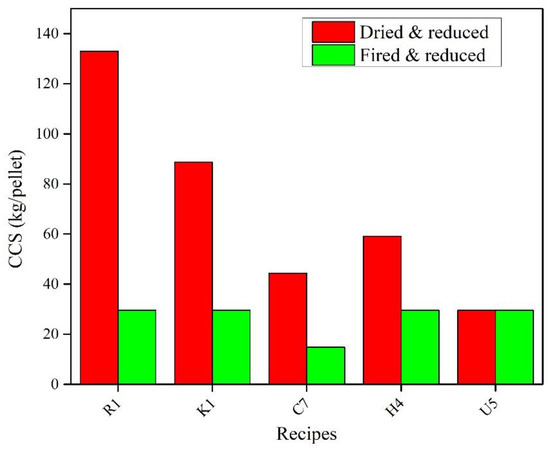
Figure 9. CCS of selected reduced dried and fired pellets.
3.7. Potential Saving of Bentonite
To summarize, a preliminary assessment of the potential bentonite savings by replacing the reference bentonite-based recipes with the top-performing recipes based on each of the four selected organic binders was performed. However, to gain more comprehensive insights, a more detailed techno-economic analysis is required.
Based on the estimated demand of iron pellets (540 Mt) by the year 2027 [4,5], Table 4 reveals that substituting the reference recipe (R1), which is based on bentonite, with the KemPel recipe (K1) would result in a reduction of 3.97 million tons of bentonite consumption. On the other hand, if we replace the same bentonite-based recipe (R1) with any of the other three organic binder-based recipes (C7, U5, H4), the consumption of bentonite could be decreased by 5.4 million tons, as none of these organic binder-based recipes contain bentonite.
Table 4. Amount of Bentonite Saved.
| Recipe | Binder | Amount of Organic Binder Required (Million Tons) | Amount of Bentonite Required (Million Tons) | Amount of Bentonite Saved (Million Tons) |
|---|---|---|---|---|
| R1 | Bentonite (1%) | 0 | 5.4 | 0 |
| K1 | KemPel (0.1%) + Bentonite (0.265%) | 0.54 | 1.43 | 3.97 |
| C7 | Alcotac CS (0.75%) | 4.05 | 0 | 5.4 |
| U5 | CMC (0.5%) | 2.7 | 0 | 5.4 |
| H4 | Alcotac FE 16 (0.5%) | 2.7 | 0 | 5.4 |
4. Conclusions
Since iron ore pellets are a crucial feed material for the DRI process, enhancing the properties of the pellets and minimizing the amount of slag produced is imperative. With the replacement of bentonite with an organic binder, the amount of slag generated would reduce significantly, resulting in a product of greater quality while decreasing the energy consumption. The current study involved the utilization of four organic binders for the pelletization of magnetite concentrate in comparison with bentonite. The major conclusions inferred from this work are as follows:
• CMC gave the best dry strength results among the four organic binders as well as bentonite. A full replacement of bentonite with 0.5 wt.% of CMC showed very promising results. Alcotac CS and Alcotac FE16 showed good dry strength-wise results comparable to the reference recipe with full replacement of bentonite with 0.75 wt. % and 0.5 wt.% of the organic binders, respectively. KemPel showed good dry strength-wise results comparable to that of the reference recipe by replacing 73.5% of bentonite with 0.1 wt.% of the organic binder. The Alcotac CS and Alcotac FE16 recipes had the best yields among the binders.
• The recipe (K1) in which 73.5% of bentonite was replaced with 0.1 wt.% of KemPel showed very good results after firing and reduction with H2, which were almost the same as the reference recipe (R1). Similarly, both Alcotac CS (C7) and Alcotac FE 16 (H4) showed lesser strength than the reference (R1) and the KemPel recipe (K1) but better results than the CMC recipe (U5). Recipe (U5) with CMC, which showed the best dry pellet strength, did not perform well after the oxidation and reduction trials.
• The dry pellets bonded with organic materials exhibited the highest reduction extent, with the order being U5 > C7 > H4 > K1 > R1. This was attributed to the porous structure resulting from the thermal decomposition of the organic binder, which facilitated H2 diffusion and enhanced pellet reduction. Conversely, the organic-bonded fired pellets had a lower reduction rate compared to the bentonite-bonded fired pellets due to the formation of magnesioferrites, which hindered the reduction process by creating a hard reducible magnesium iron oxide phase (MgO0.77FeO0.23).
As per the results, the optimal recipe was K1, which utilized a partial replacement of bentonite with 0.1 wt.% of KemPel. Moreover, when employing organic binders with the complete replacement of bentonite, recipe H4, bonded by 0.5 wt.% Alcotac FE16, demonstrated superior properties, exhibiting a high CCS compared to all other organic-bonded pellets, both before and after reduction for both dried and fired pellets.
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Parathodiel, H., Mousa, E., Ahmed, H., Elsadek, M., Forsberg, K., & Andersson, C. Developing Iron Ore Pellets Using Novel Binders for H2-Based Direct Reduction. Sustainability, 15(14), 11415. https://doi.org/10.3390/su151411415




