Carbon-Containing Pellets with Laterite Nickel Ore
Nickel, as an important strategic metal material, has various advantages such as corrosion resistance, oxidation resistance, high temperature resistance, high strength and good ductility, and is widely used in modern industry. Nickel is mainly used to produce stainless steel. 65% of the world's total nickel consumption is used to produce stainless steel and 12% is used to produce heat-resistant alloys and non-ferrous metal alloys. It is an indispensable and important metal. At present, the known nickel reserves in the world are about 160 million tons, of which nickel sulfide ore accounts for about 30% and laterite nickel ore for about 70%. Due to the low nickel content in laterite nickel ore, difficult recovery and poor economic benefits, the nickel produced by laterite nickel ore accounts for only 42% of the world's nickel output. However, in the long run, laterite nickel ore will be the main source of nickel in the future. Therefore, speeding up the research on laterite nickel ore smelting process has become one of the major international metallurgical problems and is related to the global problem of stable nickel supply.
The laterite nickel ore located in the upper part of the deposit has high contents of iron and cobalt and low contents of nickel, silicon and magnesium, which is suitable for hydrometallurgical treatment. The low iron humus laterite nickel ore and silicon magnesium laterite nickel ore located at the lower part of the deposit have high nickel, silicon and magnesium contents and low iron and cobalt contents, which are suitable for pyrometallurgical treatment. Nickel ore located in the middle of the ore bed can be treated by pyrometallurgical process or wet process. At present, 40% of the proven laterite nickel ore resources are low iron humus soil type and silicon magnesium type laterite nickel ore suitable for pyrometallurgical treatment.
Based on coal-based direct reduction process, carbon-containing pellets are prepared by cold-setting pellet method, using laterite nickel ore as the main raw material, pulverized coal as reducing agent and pure CaO as flux.
Experimental Raw Materials and Schemes
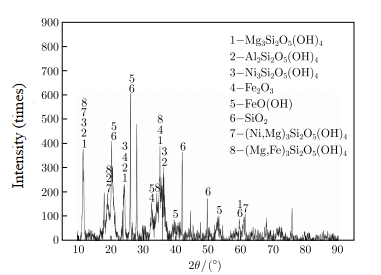
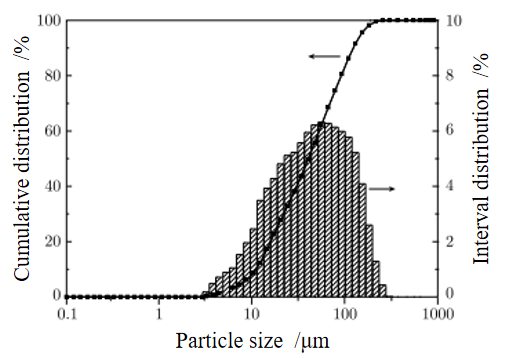
Fig 1: XRD pattern of the laterite nickel ore Fig 2: Size distribution of the laterite nickel ore
1. Experimental raw materials
(1) Laterite nickel ore. The ore powder used in this experiment is Indonesia laterite nickel ore. The laterite nickel ore has high silicon and magnesium contents and low iron and cobalt contents. It belongs to the silicon-magnesium laterite nickel ore and is a typical metamorphic peridotite, which is suitable for pyrometallurgical smelting. The main components of the laterite nickel ore are clinochrysotile (Mg3Si2O5 (OH) 4), nickel chrysotile (Ni3Si2O5 (OH) 4), goethite (FeO (OH)), hematite (Fe2O3), etc. Nickel is mainly distributed in silicate minerals and goethite. Nickel mainly replaces magnesium in silicate minerals and iron in goethite. Iron is mainly distributed in goethite, hematite and silicate minerals. Gangue minerals and kaolinite are both aqueous silicate minerals, while goethite contains crystal water, so the crystal water content of laterite nickel ore is relatively high.
The laterite nickel ore is dried in a constant temperature drying oven, crushed and ground to be used for briquetting. The particle size of laterite nickel ore powder is analyzed by LMS-30 laser particle size distribution tester. As shown in Fig. 2, it can be seen from the results that the particle size range of laterite ore powder is 3-300 µ m, and is mainly concentrated between 20-160 µ m, of which less than 200 mesh ore powder accounts for about 60% of the total mass of ore powder.
(2) Reducing agent. There are two kinds of reducing agents used in the experiment, Yangquan anthracite and Shenmu bituminous coal respectively. Before the pulverized coal is mixed with laterite mineral powder, it is dried in a constant temperature drying oven to remove its free water, and then crushed and ground to the specified particle size.
(3) Flux. As the mineral powder contains certain sulfur, when pulverized coal is added, some sulfur will also be brought in. In order to smoothly carry out the desulfurization process in the subsequent process and adjust the requirements of slag composition, a certain amount of flux needs to be added. Here we choose CaO as flux.
(4) Binder. In order to make the ball pressing process proceed smoothly and improve the strength of the pellets, it is usually necessary to add a certain amount of binder to the mixture. We chose bentonite as the binder.
2. Experimental scheme
The laterite nickel ore is dried in a constant temperature drying oven, the free water in the laterite nickel ore is fully removed, and the laterite nickel ore is crushed and ground to an appropriate particle size. After the reductant is dried, it is crushed by a sealed prototype and screened by sieves with different particle sizes to obtain pulverized coal with particle sizes of 37.5-75 µ m, 75-150 µ m and 150-300 µ m respectively. According to the pre-specified scheme, a certain amount of laterite nickel ore, reducing agent, flux, binder and water are weighed respectively, and added into the mixing mill. After being fully mixed evenly, the laterite nickel ore is fed into the counter-roller ball pressing machine and pressed into oval pellets of 40mm×25mm×20mm. The compressive strength and falling strength of the pellets were tested. The drop strength detection is to place the pellet at a height of 1.0 m so that it can freely fall on a 10mm thick steel plate, measure the drop times when the pellet breaks, measure 10 pellets respectively, and obtain the average value of the drop times as the drop strength of the pellets. Compressive strength detection is to place the pellets on a pressure testing machine, slowly pressurize them, measure the pressure value when the pellets break, measure 10 pellets respectively, and obtain the average value of the pressure as the compressive strength.




